This week’s blog post comes from Professor Chris Sorrell (Carl’s brother), who is the Professor of Ceramic Engineering at UNSW Sydney in Australia.
Chris graduated with B.S. degrees in Ceramic Engineering (1977) and Chemistry (1980) from the University of Missouri-Rolla (now the Missouri University of Science and Technology), where he gained his engineering skills.
He obtained his M.S. in Ceramic Science from Penn State University (1980), where he gained his science skills.
Finally, he obtained his Ph.D. in Ceramic Engineering (1987) from the University of New South Wales, where he brought the two sets of skills together.
This post describes work that has been published in technical papers and bulletins, references to which are included at the end.
The Grant Application
Over 10 years ago, as an academic staff member of the School of Materials Science and Engineering, University of New South Wales, I refereed an internal grant application from one of our School’s postdoctoral fellows.
In it, he proposed to make refractories from fly ash.
I criticised the application by saying, “Unless he’s not telling me something, this is a bad proposal. Fly ash is mostly glass and glass in refractories is bad news.”
As my influence was non-existent, the committee awarded the grant.
About 6 months later, the same postdoctoral fellow asked me if I would speak to his industrial partner – although he didn’t tell me why.
When I met with the two of them, the industry partner bemoaned one of my academic colleagues, who was supervising the postdoctoral fellow on a fly ash development project that he was sponsoring and asked if I would help him.
I told him that I would take on the project if my colleague agreed to a transfer of supervision.
When I explained to my colleague that the industry partner wanted to bail but was willing to leave behind an unspent $70,000 in funding, agreement was given.
The R&D Project
The R&D project on using fly ash for refractory purposes had been in motion for 6 months.
When I asked to see the data that had been generated, I noted that considerable work had been done, using a large group of fly ashes.
The general goal was to characterize them, including chemical analyses, and to use this information to make blends with alumina to land on a viable mullite composition.
This was logical because fly ash consists largely of single-crystal mullite needles in a siliceous vitreous matrix and a quick examination of the literature showed that blending with alumina was an established approach to synthesizing mullite.
Nevertheless, I concluded that a few changes in direction were needed.
First, a company intending to develop and secure intellectual property is unlikely to succeed by following conventional approaches.
Second, experimentation never should start with blends; it always should start with the end-members to establish baseline behavior.
Third, some basic ceramic testing procedures had not been followed – in this case, measuring firing shrinkage.
The Experimentation
Since considerable work had gone into identifying suitable firing temperatures, this variable was well in hand.
However, in common with all the published studies, only firing times of up to 2 hours had been examined.
Furthermore, among all the published studies of fly ash and alumina blends, not one had reported firing shrinkages.
Consequently, the first instructions that I gave to the postdoctoral fellow were to fire some pure fly ashes at the chosen temperature for 1, 2, 4, and 8 hours and to measure the firing shrinkages.
What I expected to see was one of the blue curves shown in Figure 1, below.

Figure 1: Firing Shrinkage Behavior versus Firing Time
By the 8-hour time point, the results were as expected. So, the firing time was extended to 12 hours.
At this point, we were surprised to see that no further shrinkage occurred.
The firing times were increased to 24 hours and 48 hours but, again, no further shrinkage was observed, as shown by the red curve in Figure 1.
With most of the fly ashes that we tested subsequently, the inflection point to nil-shrinkage occurred after a mere 2 hours.
This was the eureka moment.
What had happened was that the single-crystal mullite needles had grown to the extent that they had become percolated – forming an interconnected network inside the composite – a process that is well known in porcelain but occurs irregularly throughout the volume.
None of the published studies on producing mullite from fly ash had examined volumetric behavior (viz., firing shrinkage).
What we had discovered was an in situ composite synthesised largely from waste material, capable of being produced in bulk form, with nearly complete densification, and capable of performing at mullite performance temperatures.
Everyone working with this material had missed this behavior because (1) they did not examine pure fly ash, (2) they did not extend the firing time beyond 2 hours, and (3) they did not measure firing shrinkages.
The importance of starting with pure fly ash became clear as subsequent work on blends showed that excessive alumina additions increased the glass viscosity.
When the glass becomes too viscous, it no longer allows sufficient diffusion for mullite needle growth nor the large amount of matrix deformation needed for the needles to grow.
The failure to heat beyond 2 hours and thus allow energy considerations to dictate experimentation meant that percolation never was observed.
And failure to measure the firing shrinkages meant that, even if the firing times had been extended, volumetric percolation would not have been detected.
The Novel Composite
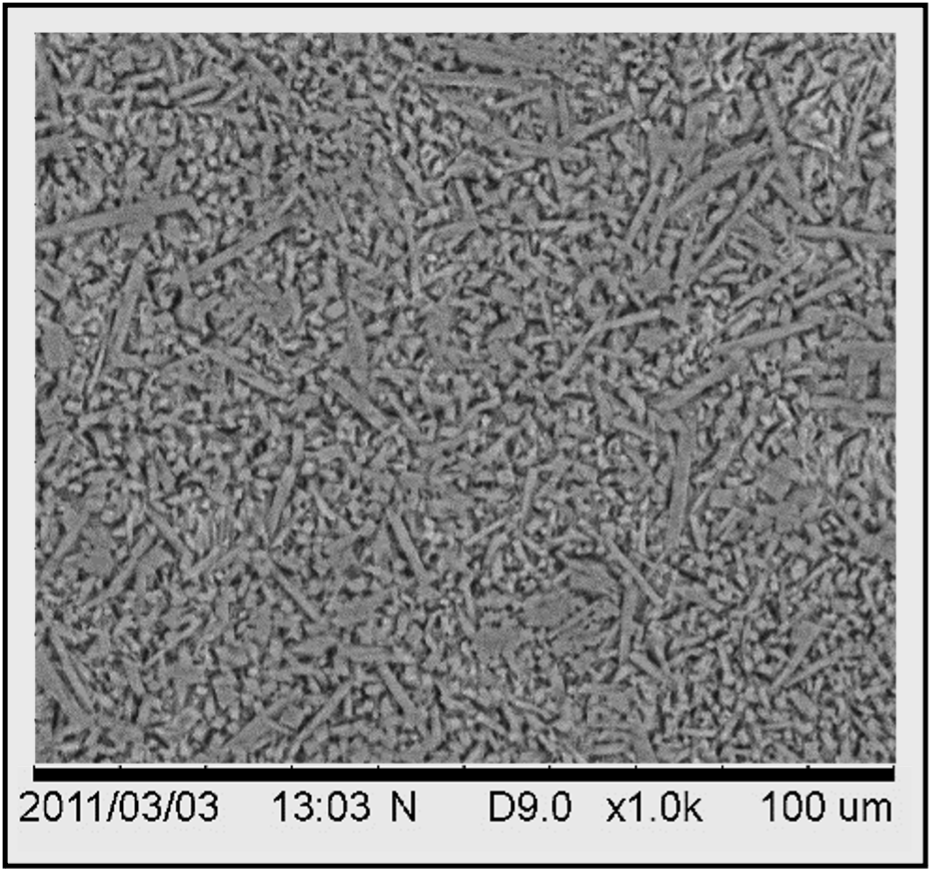
Unlike nearly all metal matrix and ceramic matrix composites, this one contains a single-crystal reinforcing phase, which is shown in Figure 2.

Figure 2. SEM micrograph (1 mm field of view)
Consequently, it offers considerably superior mechanical properties than its polycrystalline analogues.
It does not undergo recrystallization, grain growth, and consequent embrittlement with extended heating. And, of course, it goes without saying that it does not undergo any additional firing shrinkage.
How many refractories can one say this of?
What about residual glass? If there were any, it would be isolated in the triple points, so it would not cause softening owing to the percolated mullite network.
Further, because mullite is not a stoichiometric compound, it exhibits solid solubility – i.e., it can dissolve the residual glass into its matrix.
In effect, the densification process becomes one of transient liquid-phase sintering.
And, as for corrosion, the composite is >99% dense and so penetration of liquids and gases will not take place.
We subsequently obtained optical dilatometry data up to the instrument limit of 1600°C and, even at this temperature, there was no shrinkage.
While all other oxide refractories exhibit inflections indicating the onset of deformation at or below this temperature, the percolated mullite showed no indication of such a reversal.
Consequently, it is possible that this material may exhibit the intrinsic behavior of mullite, which includes a melting point of 1850°C.
The results obtained from this one simple starting point, which was nothing more than a standard exercise in materials engineering, are four patents: C.C. Sorrell, P. Koshy, and S.A. Koszo, Percolated Mullite and a Method of Forming Same issued by Australia (Patent No. 2012350159, 12 July 2012), IPCT (Patent No. WO/2013/082670, 13 June 2013), Europe (Patent No. EP2788301, 15 October 2014) and the USA (Patent No. US 2015/0344371, 3 December 2015).
The patent applications were filed by UNSW and the costs were covered by the industrial partner as part of its long-term industrial support, which has now exceeded a decade (and still continues).
To say that it is rare to receive significant industrial sponsorship over such a period would be putting it mildly. The company has invested over US$1 million and provided the financial leverage to obtain two major grants from the Australian Research Council.
While these and other developments were taking place, the postdoctoral fellow and I were able to expand a small research group working in limited areas into one of the major research groups at our institution, with over two dozen people working across a range of fields.
Most pleasingly, I have watched my co-worker develop from a green Ph.D. graduate into a sophisticated supervisor and manager of students and research projects.
The Moral of the Story
So, what is the moral of this tale?
There are several:
- Don’t dismiss concepts that may fly in the face of professional common sense as this may cause you to miss a novel outcome.
- Don’t slavishly follow what others have done as this is unlikely to lead to original results.
- Appreciate that product development starts at the most basic level, which includes simple measurements that may not be attractive for technical publications.
- A corollary to the previous point is that good research does not necessarily equate to getting published in top journals. Ultimately, good research is that which provides a benefit, whether to knowledge, a person, an institution, or a company.
- The combination of solid engineering skills with an understanding of the scientific principles behind fundamental phenomena is a powerful tool to identify and appreciate novel outcomes.
- Small projects can grow into large ones if the funding partner is shown the value of them and has the perception to understand.
- Successful research doesn’t depend only on good ideas and outcomes. It also depends on having good people, whose skills can be recognized, developed, and harnessed as key aspects of their professional development.
References
- Koshy, P.; Ho, N.; Zhong, V.; Schreck, L.; Koszo, S.A.; Severin, E.J.; Sorrell, C.C., “Fly Ash Utilisation in Mullite Fabrication: Development of Novel Percolated Mullite”, Minerals 2021, Vol. 11, No. 1, Paper 84 {16 pp.}. https://doi.org/10.3390/min11010084
- Koshy, P.; Koszo, S.A.; Severin, E.J.; Sorrell, C.C.; “High-Performance Refractory Ceramics of Percolated Mullite from Waste Materials”, American Ceramic Society Bulletin 2018, Vol. 97, No. 6, pp. 29-34.